|
POTASSIUM CARBONATE |
||
|
PRODUCT IDENTIFICATION |
||
| CAS NO. |
584-08-7 |
|
| EINECS NO. |
209-529-3 |
|
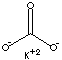
| FORMULA |
K2CO3 |
|
| MOL WT. | 138.21 | |
|
H.S. CODE |
2836.40 | |
| TOXICITY | Oral rat LD50: 1870 mg/kg | |
| SYNONYMS | Potash; Salt of Tartar; Carbonic acid, Dipotassium salt; | |
| Potassium carbonate (2:1); Kaliumcarbonat; Pearl ash; | ||
| SMILES |
by the reaction of carbon dioxide with potassium chloride + magnesium oxide (Engel-Precht process) or electrolytic caustic lye. |
|
|
CLASSIFICATION |
|
|
|
PHYSICAL AND CHEMICAL PROPERTIES |
||
| PHYSICAL STATE |
white powder or granular |
|
| MELTING POINT |
891 C |
|
| BOILING POINT | Decomposes | |
| SPECIFIC GRAVITY | 2.29 | |
| SOLUBILITY IN WATER |
soluble (insoluble in alcohol) |
|
| pH | 11.6 (Aqueous solution) | |
| VAPOR DENSITY | ||
|
AUTOIGNITION |
|
|
|
NFPA RATINGS |
Health: 2 Flammability: 0 Reactivity: 1 | |
|
REFRACTIVE INDEX |
|
|
| FLASH POINT | Not considered to be a fire hazard | |
| STABILITY | Stable under ordinary conditions | |
|
APPLICATIONS |
||
|
Potassium Carbonate is used in glasses, ceramics, explosives, fertilizers,and glazes industry, in the the manufacture of soft soaps, inorganic salts, and in dyeing and wool finishing. |
||
| SALES SPECIFICATION | ||
|
APPEARANCE |
white powder |
|
| ASSAY |
98.5% min |
|
| CHLORIDES |
0.1% max |
|
| SULPHATES |
0.1% max |
|
| Fe |
0.003% max |
|
| WATER INSOLUBLES |
0.05% max |
|
| HEAVY METALS |
10ppm max |
|
| MEAN PARTICLE SIZE |
0.25 - 1mm |
|
| TRANSPORTATION | ||
| PACKING | 50kgs in bag | |
| HAZARD CLASS | listed on the TSCA inventory | |
| UN NO. | ||
| REMARKS | ||
|
Commercially anhydrous and 1.5 hydrated forms are available. K2CO3 (melting point at 891 C) decomposes before boiled. 2K2CO3.3H2O dehydrates to K2CO3.H2O at 100 C and to K2CO3 at 130 C. |
||